UNCW pauses COVID-19 vaccination clinic
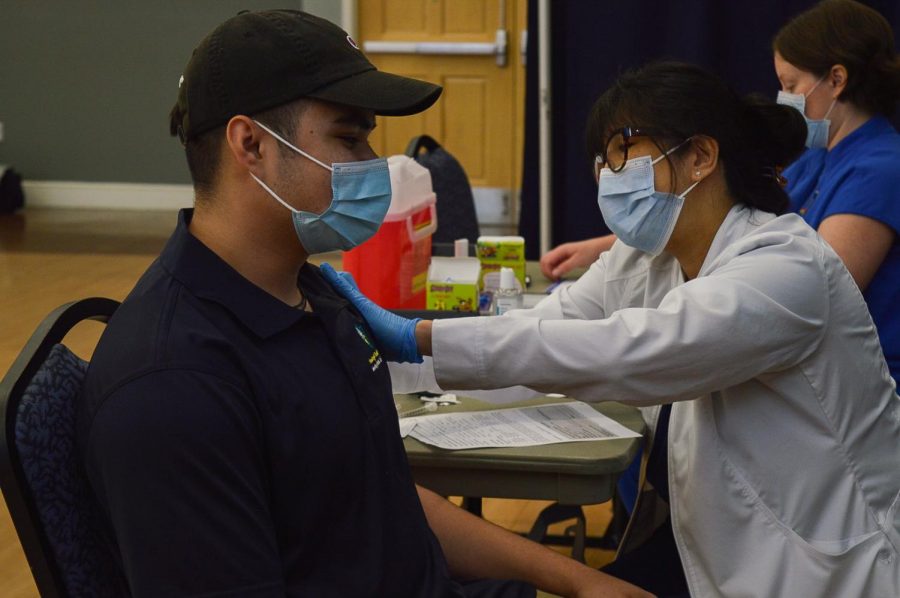
UNCW student, Alfredo Espinal, receives his vaccine.
UNC Wilmington (UNCW) has paused operations at their on-campus vaccination clinic effective immediately and until further notice.
The pause is due to guidance from the Centers for Disease Control and Prevention (CDC), the Food and Drug Administration (FDA) and state guidance. On April 13, both the CDC and FDA issued a pause on all administering of the Johnson & Johnson vaccine due to reports of rare blood clots.
“Out of more than 6.8 million doses of the vaccine administered in the U.S., six recipients reported rare blood clots and symptoms occurred six to 13 days after vaccination. A joint statement from the FDA and the CDC provides more information here. To date, UNCW has not received any reports of similar health concerns among recipients vaccinated at the campus clinic,” as written in the announcement.
The CDC’s advisory committee on immunization practices (ACIP) is set to meet on April 14 to discuss and review the six reported cases of blood clots. Meanwhile, the FDA will also review their findings as it investigates the cases.
Since its opening, UNCW has only been administering the Johnson & Johnson vaccine. According to a university spokesperson, UNCW has administered just over 2,500 doses.
The university will share updates about the university clinic’s operations and appointment rescheduling as information becomes available.
If you experience a severe headache, abdominal pain, leg pain, or shortness of breath within three weeks after receiving the Johnson & Johnson vaccine, please contact your healthcare provider.